Español – Chem Fatale Informe
Chem Fatale Report:
Potential Health Effects of Toxic Chemicals in Feminine Care Products
 Feminine care. Feminine hygiene. Personal cleansing products. Intimate care. No matter what you call them, these consumer products are manufactured for and marketed exclusively to women. The purpose of feminine care products is to clean, moisturize, absorb discharge or otherwise treat the sensitive skin and tissues of the vaginal area. Women are told they are necessary for personal hygiene, a “fresher feeling,” or “greater confidence,” and the companies marketing these products imply that this improved cleanliness will promote good health and increase sex appeal.
Feminine care. Feminine hygiene. Personal cleansing products. Intimate care. No matter what you call them, these consumer products are manufactured for and marketed exclusively to women. The purpose of feminine care products is to clean, moisturize, absorb discharge or otherwise treat the sensitive skin and tissues of the vaginal area. Women are told they are necessary for personal hygiene, a “fresher feeling,” or “greater confidence,” and the companies marketing these products imply that this improved cleanliness will promote good health and increase sex appeal.
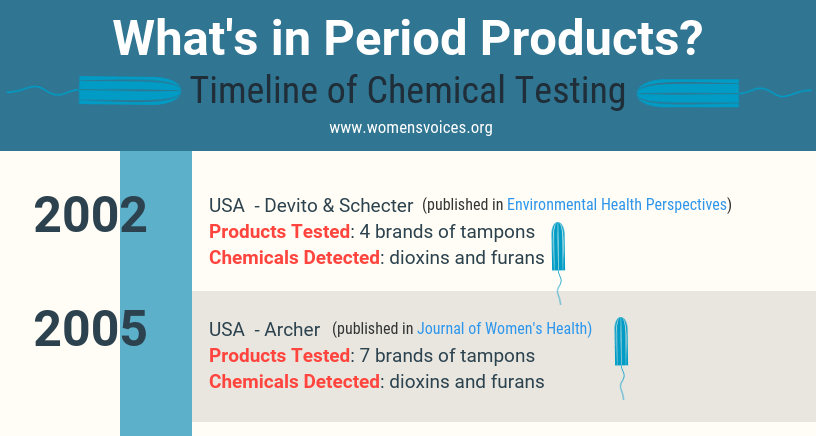
A closer look at the impacts of these products, and the chemicals they contain, tell a much different story. Products intended for use on or in an incredibly absorbent part of a woman’s body are marketed and sold with little to no data assuring the ingredients they contain are safe. Ingredients are determined “safe,” operating under the premise that they are used on ordinary skin just like other cosmetic products. That means chemicals of concern such as carcinogens, reproductive toxins, endocrine disruptors, and allergens are being used on, or even in, the extremely permeable mucus membranes of the vaginal area.
Feminine care products are widely used by women in the United States and constitute a $3 billion dollar industry. The most popular feminine care products are tampons and menstrual pads, used by 70-85 percent of women. Douches, sprays, washes, and wipes are used by a smaller percentage of women (approximately 10-40 percent), with rates considerably higher among African-American, Latina and low-income women. This report highlights the potential health concerns related to toxic and allergenic chemicals found in feminine care products and outlines the considerable data gaps in our knowledge about them. These products, and their ingredients, require both more research, and greater scrutiny to ensure the safety of their use.